The first large-scale nuclear reactors were built in 1944 at Hanford, Washington, for the production of nuclear weapons material. The fuel was natural uranium metal; the moderator, graphite. Plutonium was produced in these plants by neutron absorption in uranium-238; the power produced was not used.
Light-Water and Heavy-Water Reactors
A variety of reactor types, characterized by the type of fuel, moderator, and coolant used, have been built throughout the world for the production of electric power. In the United States, with few exceptions, power reactors use nuclear fuel in the form of uranium oxide isotopically enriched to about three percent uranium-235. The moderator and coolant are highly purified ordinary water. A reactor of this type is called a light-water reactor (LWR).
In the pressurized-water reactor (PWR), a version of the LWR system, the water coolant operates at a pressure of about 150 atmospheres. It is pumped through the reactor core, where it is heated to about 325° C (about 620° F). The superheated water is pumped through a steam generator, where, through heat exchangers, a secondary loop of water is heated and converted to steam. This steam drives one or more turbine generators, is condensed, and is pumped back to the steam generator. The secondary loop is isolated from the water in the reactor core and, therefore, is not radioactive. A third stream of water from a lake, river, or cooling tower is used to condense the steam. The reactor pressure vessel is about 15 m (about 49 ft) high and 5 m (about 16.4 ft) in diameter, with walls 25 cm (about 10 in) thick. The core houses some 82 metric tons of uranium oxide contained in thin corrosion-resistant tubes clustered into fuel bundles.
In the boiling-water reactor (BWR), a second type of LWR, the water coolant is permitted to boil within the core, by operating at somewhat lower pressure. The steam produced in the reactor pressure vessel is piped directly to the turbine generator, is condensed, and is then pumped back to the reactor. Although the steam is radioactive, there is no intermediate heat exchanger between the reactor and turbine to decrease efficiency. As in the PWR, the condenser cooling water has a separate source, such as a lake or river.
The power level of an operating reactor is monitored by a variety of thermal, flow, and nuclear instruments. Power output is controlled by inserting or removing from the core a group of neutron-absorbing control rods. The position of these rods determines the power level at which the chain reaction is just self-sustaining.
During operation, and even after shutdown, a large, 1,000-megawatt (MW) power reactor contains billions of curies of radioactivity. Radiation emitted from the reactor during operation and from the fission products after shutdown is absorbed in thick concrete shields around the reactor and primary coolant system. Other safety features include emergency core cooling systems to prevent core overheating in the event of malfunction of the main coolant systems and, in most countries, a large steel and concrete containment building to retain any radioactive elements that might escape in the event of a leak.
Although more than 100 nuclear power plants were operating or being built in the United States at the beginning of the 1980s, in the aftermath of the Three Mile Island accident in Pennsylvania in 1979 safety concerns and economic factors combined to block any additional growth in nuclear power. No orders for nuclear plants have been placed in the United States since 1978, and some plants that have been completed have not been allowed to operate. In 1996 about 22 percent of the electric power generated in the United States came from nuclear power plants. In contrast, in France almost three-quarters of the electricity generated was from nuclear power plants.
In the initial period of nuclear power development in the early 1950s, enriched uranium was available only in the United States and the Union of Soviet Socialist Republics (USSR). The nuclear power programs in Canada, France, and the United Kingdom therefore centered about natural uranium reactors, in which ordinary water cannot be used as the moderator because it absorbs too many neutrons. This limitation led Canadian engineers to develop a reactor cooled and moderated by deuterium oxide (D2O), or heavy water. The Canadian deuterium-uranium reactor known as CANDU has operated satisfactorily in Canada, and similar plants have been built in India, Argentina, and elsewhere.
In the United Kingdom and France the first full-scale power reactors were fueled with natural uranium metal, were graphite-moderated, and were cooled with carbon dioxide gas under pressure. These initial designs have been superseded in the United Kingdom by a system that uses enriched uranium fuel. In France the initial reactor type chosen was dropped in favor of the PWR of U.S. design when enriched uranium became available from French isotope-enrichment plants. Russia and the other successor states of the USSR had a large nuclear power program, using both graphite-moderated and PWR systems.
 |
Light Water Reactors
 |
Heavy Water Reactors
|
|
Propulsion reactors
Nuclear power plants similar to the PWR are used for the propulsion plants of large surface naval vessels such as the aircraft carrier USS Nimitz. The basic technology of the PWR system was first developed in the U.S. naval reactor program directed by Admiral Hyman G. Rickover. Reactors for submarine propulsion are generally physically smaller and use more highly enriched uranium to permit a compact core. The United States, the United Kingdom, Russia, and France all have nuclear-powered submarines with such power plants.
Three experimental seagoing nuclear cargo ships were operated for limited periods by the United States, Germany, and Japan. Although they were technically successful, economic conditions and restrictive port regulations brought an end to these projects. The Soviet government built the first successful nuclear-powered icebreaker, Lenin, for use in clearing the Arctic sea-lanes.
Research Reactors
A variety of small nuclear reactors have been built in many countries for use in education and training, research, and the production of radioactive isotopes. These reactors generally operate at power levels near one MW, and they are more easily started up and shut down than larger power reactors.
A widely used type is called the swimming-pool reactor. The core is partially or fully enriched uranium-235 contained in aluminum alloy plates, immersed in a large pool of water that serves as both coolant and moderator. Materials may be placed directly in or near the reactor core to be irradiated with neutrons. Various radioactive isotopes can be produced for use in medicine, research, and industry. Neutrons may also be extracted from the reactor core by means of beam tubes to be used for experimentation.
Breeder Reactors
Uranium, the natural resource on which nuclear power is based, occurs in scattered deposits throughout the world. Its total supply is not fully known, and may be limited unless sources of very low concentration such as granites and shale were to be used. Conservatively estimated U.S. resources of uranium having an acceptable cost lie in the range of two million to five million metric tons. The lower amount could support an LWR nuclear power system providing about 30 percent of U.S. electric power for only about 50 years. The principal reason for this relatively brief life span of the LWR nuclear power system is its very low efficiency in the use of uranium: only approximately one percent of the energy content of the uranium is made available in this system.
The key feature of a breeder reactor is that it produces more fuel than it consumes. It does this by promoting the absorption of excess neutrons in a fertile material. Several breeder reactor systems are technically feasible. The breeder system that has received the greatest worldwide attention uses uranium-238 as the fertile material. When uranium-238 absorbs neutrons in the reactor, it is transmuted to a new fissionable material, plutonium, through a nuclear process called ß (beta) decay. The sequence of nuclear reactions is
In beta decay a nuclear neutron decays into a proton and a beta particle (a high-energy electron).
When plutonium-239 itself absorbs a neutron, fission can occur, and on the average about 2.8 neutrons are released. In an operating reactor, one of these neutrons is needed to cause the next fission and keep the chain reaction going. On the average about 0.5 neutron is uselessly lost by absorption in the reactor structure or coolant. The remaining 1.3 neutrons can be absorbed in uranium-238 to produce more plutonium via the reactions in equation (3).
The breeder system that has had the greatest development effort is called the liquid-metal fast breeder reactor (LMFBR). In order to maximize the production of plutonium-239, the velocity of the neutrons causing fission must remain fast—at or near their initial release energy. Any moderating materials, such as water, that might slow the neutrons must be excluded from the reactor. A molten metal, liquid sodium, is the preferred coolant liquid. Sodium has very good heat transfer properties, melts at about 100° C (about 212° F), and does not boil until about 900° C (about 1650° F). Its main drawbacks are its chemical reactivity with air and water and the high level of radioactivity induced in it in the reactor.
Development of the LMFBR system began in the United States before 1950, with the construction of the first experimental breeder reactor, EBR-1. A larger U.S. program, on the Clinch River, was halted in 1983, and only experimental work was to continue. In the United Kingdom, France, and Russia and the other successor states of the USSR, working breeder reactors were installed, and experimental work continued in Germany and Japan.
In one design of a large LMFBR power plant, the core of the reactor consists of thousands of thin stainless steel tubes containing mixed uranium and plutonium oxide fuel: about 15 to 20 percent plutonium-239, the remainder uranium. Surrounding the core is a region called the breeder blanket, which contains similar rods filled only with uranium oxide. The entire core and blanket assembly measures about 3 m (about 10 ft) high by about 5 m (about 16.4 ft) in diameter and is supported in a large vessel containing molten sodium that leaves the reactor at about 500° C (about 930° F). This vessel also contains the pumps and heat exchangers that aid in removing heat from the core. Steam is produced in a second sodium loop, separated from the radioactive reactor coolant loop by the intermediate heat exchangers in the reactor vessel. The entire nuclear reactor system is housed in a large steel and concrete containment building.
The first large-scale plant of this type for the generation of electricity, called Super-Phénix, went into operation in France in 1984. (However, concerns about operational safety and environmental contamination led the French government to announce in 1998 that Super-Phénix would be dismantled). An intermediate-scale plant, the BN-600, was built on the shore of the Caspian Sea for the production of power and the desalination of water. The British have a large 250-MW prototype in Scotland. The LMFBR produces about 20 percent more fuel than it consumes. In a large power reactor enough excess new fuel is produced over 20 years to permit the loading of another similar reactor. In the LMFBR system about 75 percent of the energy content of natural uranium is made available, in contrast to the one percent in the LWR
NUCLEAR FUELS AND WASTES
The Nuclear Fuel Cycle
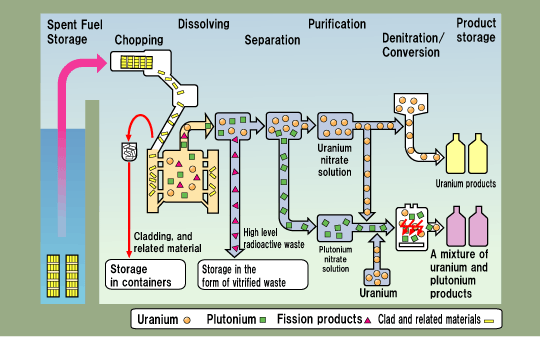
The hazardous fuels used in nuclear reactors present handling problems in their use. This is particularly true of the spent fuels, which must be stored or disposed of in some way.
Any electric power generating plant is only one part of a total energy cycle. The uranium fuel cycle that is employed for LWR systems currently dominates worldwide nuclear power production and includes many steps. Uranium, which contains about 0.7 percent uranium-235, is obtained from either surface or underground mines. The ore is concentrated by milling and then shipped to a conversion plant, where its elemental form is changed to uranium hexafluoride gas (UF6). At an isotope enrichment plant, the gas is forced against a porous barrier that permits the lighter uranium-235 to penetrate more readily than uranium-238. This process enriches uranium to about 3 percent uranium-235. The depleted uranium—the tailings—contain about 0.3 percent uranium-235. The enriched product is sent to a fuel fabrication plant, where the UF6 gas is converted to uranium oxide powder, then into ceramic pellets that are loaded into corrosion-resistant fuel rods. These are assembled into fuel elements and are shipped to the reactor power plant. The world’s supply of enriched uranium fuel for powering commercial nuclear power plants is produced by five consortiums located in the United States, Western Europe, Russia, and Japan. The United States consortium—the federally owned United States Enrichment Corporation—produces 40 percent of this enriched uranium.
A typical 1,000-MW pressurized-water reactor has about 200 fuel elements, one-third of which are replaced each year because of the depletion of the uranium-235 and the buildup of fission products that absorb neutrons. At the end of its life in the reactor, the fuel is tremendously radioactive because of the fission products it contains and hence is still producing a considerable amount of energy. The discharged fuel is placed in water storage pools at the reactor site for a year or more.
At the end of the cooling period the spent fuel elements are shipped in heavily shielded casks either to permanent storage facilities or to a chemical reprocessing plant. At a reprocessing plant, the unused uranium and the plutonium-239 produced in the reactor are recovered and the radioactive wastes concentrated. (In the late 1990s neither such facility was yet available in the United States for power plant fuel, and temporary storage was used.)
The spent fuel still contains almost all the original uranium-238, about one-third of the uranium-235, and some of the plutonium-239 produced in the reactor. In cases where the spent fuel is sent to permanent storage, none of this potential energy content is used. In cases where the fuel is reprocessed, the uranium is recycled through the diffusion plant, and the recovered plutonium-239 may be used in place of some uranium-235 in new fuel elements. At the end of the 20th century, no reprocessing of fuel occurred in the United States because of environmental, health, and safety concerns, and the concern that plutonium-239 could be used illegally for the manufacture of weapons.
In the fuel cycle for the LMFBR, plutonium bred in the reactor is always recycled for use in new fuel. The feed to the fuel-element fabrication plant consists of recycled uranium-238, depleted uranium from the isotope separation plant stockpile, and part of the recovered plutonium-239. No additional uranium needs to be mined, as the existing stockpile could support many breeder reactors for centuries. Because the breeder produces more plutonium-239 than it requires for its own refueling, about 20 percent of the recovered plutonium is stored for later use in starting up new breeders. Because new fuel is bred from the uranium-238, instead of using only the natural uranium-235 content, about 75 percent of the potential energy of uranium is made available with the breeder cycle.
The final step in any of the fuel cycles is the long-term storage of the highly radioactive wastes, which remain biologically hazardous for thousands of years. Fuel elements may be stored in shielded, guarded repositories for later disposition or may be converted to very stable compounds, fixed in ceramics or glass, encapsulated in stainless steel canisters, and buried far underground in very stable geologic formations. However, the safety of such repositories is the subject of public controversy, especially in the geographic region in which the repository is located or is proposed to be built. For example, environmentalists plan to file a lawsuit to close a repository built near Carlsbad, New Mexico. In 1999, this repository began receiving shipments of radioactive waste from the manufacture of nuclear weapons in United States during the Cold War. Another controversy centers around a proposed repository at Yucca Mountain, Nevada. Opposition from state residents and questions about the geologic stability of this site have helped prolong government studies. Even if opened, the site will not receive shipments of radioactive waste until at least 2010.

Nuclear Safety
Public concern about the acceptability of nuclear power from fission arises from two basic features of the system. The first is the high level of radioactivity present at various stages of the nuclear cycle, including disposal. The second is the fact that the nuclear fuels uranium-235 and plutonium-239 are the materials from which nuclear weapons are made.
U.S. President Dwight D. Eisenhower announced the U.S. Atoms for Peace program in 1953. It was perceived as offering a future of cheap, plentiful energy. The utility industry hoped that nuclear power would replace increasingly scarce fossil fuels and lower the cost of electricity. Groups concerned with conserving natural resources foresaw a reduction in air pollution and strip mining. The public in general looked favorably on this new energy source, seeing the program as a realization of hopes for the transition of nuclear power from wartime to peaceful uses.
Nevertheless, after this initial euphoria, reservations about nuclear energy grew as greater scrutiny was given to issues of nuclear safety and weapons proliferation. In the United States and other countries many groups oppose nuclear power. In addition, high construction costs, strict building and operating regulations, and high costs for waste disposal make nuclear power plants much more expensive to build and operate than plants that burn fossil fuels. In some industrialized countries, the nuclear power industry has come under growing pressure to cut operating expenses and become more cost-competitive. Other countries have begun or planned to phase out nuclear power completely.
At the end of the 20th century, many experts viewed Asia as the only possible growth area for nuclear power. In the late 1990s, China, Japan, South Korea, and Taiwan had nuclear power plants under construction. However, many European nations were reducing or reversing their commitments to nuclear power. For example, Sweden committed to phasing out nuclear power by 2010. France canceled several planned reactors and was considering the replacement of aging nuclear plants with environmentally safer fossil-fuel plants. Germany announced plans in 1998 to phase out nuclear energy. In the United States, no new reactors had been ordered since 1978.
In 1996, 21.9 percent of the electricity generated in the United States was produced by nuclear power. By 1998 that amount had decreased to 20 percent. Because no orders for nuclear plants have been placed since 1978, this share should continue to decline as existing nuclear plants are eventually closed. In 1998 Commonwealth Edison, the largest private owner and operator of nuclear plants in the United States, had only four of 12 nuclear power plants online. Industry experts cite economic, safety, and labor problems as reasons for these shutdowns
Radiological Hazards
Radioactive materials emit penetrating, ionizing radiation that can injure living tissues. The commonly used unit of radiation dose equivalent in humans is the sievert. (In the United States, rems are still used as a measure of dose equivalent. One rem equals 0.01 sievert.) Each individual in the United States and Canada is exposed to about 0.003 sievert per year from natural background radiation sources. An exposure to an individual of five sieverts is likely to be fatal. A large population exposed to low levels of radiation will experience about one additional cancer for each 10 sieverts total dose equivalent.
Radiological hazards can arise in most steps of the nuclear fuel cycle. Radioactive radon gas is a colorless gas produced from the decay of uranium. As a result, radon is a common air pollutant in underground uranium mines. The mining and ore-milling operations leave large amounts of waste material on the ground that still contain small concentrations of uranium. To prevent the release of radioactive radon gas into the air from this uranium waste, these wastes must be stored in waterproof basins and covered with a thick layer of soil.
Uranium enrichment and fuel fabrication plants contain large quantities of three-percent uranium-235, in the form of corrosive gas, uranium hexafluoride, UF6. The radiological hazard, however, is low, and the usual care taken with a valuable material posing a typical chemical hazard suffices to ensure safety.
Reactor Safety Systems
The safety of the power reactor itself has received the greatest attention. In an operating reactor, the fuel elements contain by far the largest fraction of the total radioactive inventory. A number of barriers prevent fission products from leaking into the air during normal operation. The fuel is clad in corrosion-resistant tubing. The heavy steel walls of the primary coolant system of the PWR form a second barrier. The water coolant itself absorbs some of the biologically important radioactive isotopes such as iodine. The steel and concrete building is a third barrier.
During the operation of a power reactor, some radioactive compounds are unavoidably released. The total exposure to people living nearby is usually only a few percent of the natural background radiation. Major concerns arise, however, from radioactive releases caused by accidents in which fuel damage occurs and safety devices fail. The major danger to the integrity of the fuel is a loss-of-coolant accident in which the fuel is damaged or even melts. Fission products are released into the coolant, and if the coolant system is breached, fission products enter the reactor building.
Reactor systems rely on elaborate instrumentation to monitor their condition and to control the safety systems used to shut down the reactor under abnormal circumstances. Backup safety systems that inject boron into the coolant to absorb neutrons and stop the chain reaction to further assure shutdown are part of the PWR design. Light-water reactor plants operate at high coolant pressure. In the event of a large pipe break, much of the coolant would flash into steam and core cooling could be lost. To prevent a total loss of core cooling, reactors are provided with emergency core cooling systems that begin to operate automatically on the loss of primary coolant pressure. In the event of a steam leak into the containment building from a broken primary coolant line, spray coolers are actuated to condense the steam and prevent a hazardous pressure rise in the building.
Fuel Reprocessing
The fuel reprocessing step poses a combination of radiological hazards. One is the accidental release of fission products if a leak should occur in chemical equipment or the cells and building housing it. Another may be the routine release of low levels of inert radioactive gases such as xenon and krypton. In 1966 a commercial reprocessing plant opened in West Valley, New York. But in 1972 this reprocessing plant was closed after generating more than 600,000 gallons of high-level radioactive waste. After the plant was closed, a portion of this radioactive waste was partially treated and cemented into nearly 20,000 steel drums. In 1996, the United States Department of Energy began to solidify the remaining liquid radioactive wastes into glass cylinders. At the end of the 20th century, no reprocessing plants were licensed in the United States.
Of major concern in chemical reprocessing is the separation of plutonium-239, a material that can be used to make nuclear weapons. The hazards of theft of plutonium-239, or its use for intentional but hidden production for weapons purposes, can best be controlled by political rather than technical means. Improved security measures at sensitive points in the fuel cycle and expanded international inspection by the International Atomic Energy Agency (IAEA) offer the best prospects for controlling the hazards of plutonium diversion.
Waste Management
The last step in the nuclear fuel cycle, waste management, remains one of the most controversial. The principal issue here is not so much the present danger as the danger to generations far in the future. Many nuclear wastes remain radioactive for thousands of years, beyond the span of any human institution. The technology for packaging the wastes so that they pose no current hazard is relatively straightforward. The difficulty lies both in being adequately confident that future generations are well protected and in making the political decision on how and where to proceed with waste storage. Permanent but potentially retrievable storage in deep stable geologic formations seems the best solution. In 1988 the U.S. government chose Yucca Mountain, a Nevada desert site with a thick section of porous volcanic rocks, as the nation's first permanent underground repository for more than 36,290 metric tons of nuclear waste. However, opposition from state residents and uncertainty that Yucca Mountain may not be completely insulated from earthquakes and other hazards has prolonged government studies. For example, a geological study by the U.S. Department of Energy detected water in several mineral samples taken at the Yucca Mountain site. The presence of water in these samples suggests that water may have once risen up through the mountain and later subsided. Because such an event could jeopardize the safety of a nuclear waste repository, the Department of Energy has funded more study of these fluid intrusions. A U$S2 billion repository built in underground salt caverns near Carlsbad, New Mexico, is designed to store radioactive waste from the manufacture of nuclear weapons during the Cold War. This repository, located 655 meters (2,150 feet) underground, is designed to slowly collapse and encapsulate the plutonium-contaminated waste in the salt beds. Although the repository began receiving radioactive waste shipments in April 1999, environmentalists planned to file a lawsuit to close the Carlsbad repository.
NUCLEAR FUSION
The release of nuclear energy can occur at the low end of the binding energy curve through the fusion of two light nuclei into a heavier one. The energy radiated by stars, including the Sun, arises from such fusion reactions deep in their interiors. At the enormous pressure and at temperatures above 15 million ° C (27 million ° F) existing there, hydrogen nuclei combine according to equation (1) and give rise to most of the energy released by the Sun.
Nuclear fusion was first achieved on earth in the early 1930s by bombarding a target containing deuterium, the mass-2 isotope of hydrogen, with high-energy deuterons in a cyclotron . To accelerate the deuteron beam a great deal of energy is required, most of which appeared as heat in the target. As a result, no net useful energy was produced. In the 1950s the first large-scale but uncontrolled release of fusion energy was demonstrated in the tests of thermonuclear weapons by the United States, the USSR, the United Kingdom, and France. This was such a brief and uncontrolled release that it could not be used for the production of electric power.
In the fission reactions discussed earlier, the neutron, which has no electric charge, can easily approach and react with a fissionable nucleus—for example, uranium-235. In the typical fusion reaction, however, the reacting nuclei both have a positive electric charge, and the natural repulsion between them, called Coulomb repulsion, must be overcome before they can join. This occurs when the temperature of the reacting gas is sufficiently high—50 to 100 million ° C (90 to 180 million ° F). In a gas of the heavy hydrogen isotopes deuterium and tritium at such temperature, the fusion reaction
occurs, releasing about 17.6 MeV per fusion event. The energy appears first as kinetic energy of the helium-4 nucleus and the neutron, but is soon transformed into heat in the gas and surrounding materials.
If the density of the gas is sufficient—and at these temperatures the density need be only 10-5 atm, or almost a vacuum—the energetic helium-4 nucleus can transfer its energy to the surrounding hydrogen gas, thereby maintaining the high temperature and allowing subsequent fusion reactions, or a fusion chain reaction, to take place. Under these conditions, “nuclear ignition” is said to have occurred.
The basic problems in attaining useful nuclear fusion conditions are (1) to heat the gas to these very high temperatures and (2) to confine a sufficient quantity of the reacting nuclei for a long enough time to permit the release of more energy than is needed to heat and confine the gas. A subsequent major problem is the capture of this energy and its conversion to electricity.
At temperatures of even 100,000° C (180,000° F), all the hydrogen atoms are fully ionized. The gas consists of an electrically neutral assemblage of positively charged nuclei and negatively charged free electrons. This state of matter is called a plasma.
A plasma hot enough for fusion cannot be contained by ordinary materials. The plasma would cool very rapidly, and the vessel walls would be destroyed by the extreme heat. However, since the plasma consists of charged nuclei and electrons, which move in tight spirals around the lines of force of strong magnetic fields, the plasma can be contained in a properly shaped magnetic field region without reacting with material walls.
In any useful fusion device, the energy output must exceed the energy required to confine and heat the plasma. This condition can be met when the product of confinement time t and plasma density n exceeds about 1014. The relationship tn= 1014 is called the Lawson criterion.
Numerous schemes for the magnetic confinement of plasma have been tried since 1950 in the United States, Russia, the United Kingdom, Japan, and elsewhere. Thermonuclear reactions have been observed, but the Lawson number rarely exceeded 1012. One device, however—the tokamak, originally suggested in the USSR by Igor Tamm and Andrey Sakharov—began to give encouraging results in the early 1960s.
The confinement chamber of a tokamak has the shape of a torus, with a minor diameter of about 1 m (about 3.3 ft) and a major diameter of about 3 m (about 9.8 ft). A toroidal (donut-shaped) magnetic field of about 50,000 gauss is established inside this chamber by large electromagnets. A longitudinal current of several million amperes is induced in the plasma by the transformer coils that link the torus. The resulting magnetic field lines, spirals in the torus, stably confine the plasma.
Based on the successful operation of small tokamaks at several laboratories, two large devices were built in the early 1980s, one at Princeton University in the United States and one in the USSR. The enormous magnetic fields in a tokamak subject the plasma to extremely high temperatures and pressures, forcing the atomic nuclei to fuse. As the atomic nuclei are fused together, an extraordinary amount of energy is released. During this fusion process, the temperature in the tokamak reaches three times that of the Sun’s core.
Another possible route to fusion energy is that of inertial confinement. In this concept, the fuel—tritium or deuterium—is contained within a tiny glass sphere that is then bombarded on several sides by a pulsed laser or heavy ion beam. This causes an implosion of the glass sphere, setting off a thermonuclear reaction that ignites the fuel. Several laboratories in the United States and elsewhere are currently pursuing this possibility. In the late 1990s, many researchers concentrated on the use of beams of heavy ions, such as barium ions, rather than lasers to trigger inertial-confinement fusion. Researchers chose heavy ion beams because heavy ion accelerators can produce intense ion pulses at high repetition rates and because heavy ion accelerators are extremely efficient at converting electric power into ion beam energy, thus reducing the amount of input power. Also in comparison to laser beams, ion beams can penetrate the glass sphere and fuel more effectively to heat the fuel.
Progress in fusion research has been promising, but the development of practical systems for creating a stable fusion reaction that produces more power than it consumes will probably take decades to realize. The research is expensive, as well. However, some progress was made in the early 1990s. In 1991, for the first time ever, a significant amount of energy—about 1.7 million watts—was produced from controlled nuclear fusion at the Joint European Torus (JET) Laboratory in England. In December 1993, researchers at Princeton University used the Tokamak Fusion Test Reactor to produce a controlled fusion reaction that output 5.6 million watts of power. However, both the JET and the Tokamak Fusion Test Reactor consumed more energy than they produced during their operation.
If fusion energy does become practical, it offers the following advantages:
1) a limitless source of fuel, deuterium from the ocean
2) no possibility of a reactor accident, as the amount of fuel in the system is very small
3) waste products much less radioactive and simpler to handle than those from fission systems.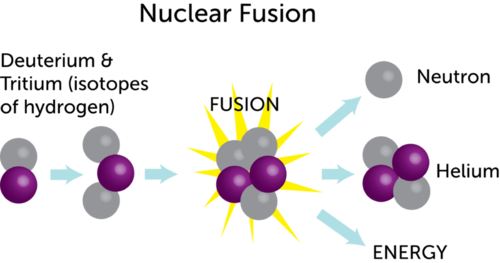
|
|
|


